| Company Name | Contact Info | Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ERASER MEDİKAL TIBBİ CİH. SAĞLIK ÜRÜN. PAZ. SAN. VE TİC. LTD. ŞTİ.
Pınar Cad. No: 78 Pınarbaşı / Bornova / İZMİR / TÜRKİYE
|
Contact Info: +90 232 479 8060
|
Hall: 3
Stand: 334A3
|
||||||||||||
| Product Groups | ||||||||||||||
|
||||||||||||||
- Company Info
- Products
- Represented Companies
- Company Brands
Eraser Medical LLC was founded in 2007 in İzmir. It started its services in the fields of preparation and administration of chemotherapy and TPN mixtures with the experience and knowledge gained by its expert staff as a result of many years in the sector. The company registered its experience with the ONCOERA brand, which it has been producing since 2014. Today, Eraser Medical is the manufacturer, authorized dealer and exporter of ONCOERA, NUTRIERA, ONCOMIX and ERAVERSE branded products. Eraser Medical LLC, which produces disposable sterile and non-sterile medical devices and medical supplies, also manufactures Chemotherapy, TPN, Aseptic Drug Preparation Devices and software, by its strong R&D infrastructure. Company has adopted a scientific-based and customer-oriented production and sales policy. Products of Eraser Medical are exported to 4 continents. Production Facility The production facility has a closed area of 1,700 m2. The production, assembly and packaging of medical devices is carried out in 2 Clean Rooms of Class 10,000 and 2 Clean Rooms of Class 100,000. The biological load on the products is kept under control in these clean rooms and sterilization safety is ensured. Disposable medical equipment production consists of injection, extrusion, assembly, packaging and sterilization sections. The storage processes of the products before loading are monitored in a 500 m2 area. The raw materials used in production are biocompatible and meet USP and ISO 10933 standards. Eraser Medical LLC has ISO 9001, ISO 13485 and CE certificates. In the production facility, ONCOERA, NUTRIERA, ONCOMIX and ERAVERSE branded products registered to Eraser Medical LLC are produced. R&D Center The R&D center is structured to develop software and device hardware and has a wide range of device park. R&D processes are advanced by channeling the years of experience of the marketing, engineering, software, medical and quality teams to a common point in order to develop technology. In research and development activities, both the needs of the sector are taken into account and the most up-to-date scientific trends in the field of health are utilized. To watch our corporate trailer ⤵️ https//www.youtube.com/watch?v=5fQuOYls4gE&t
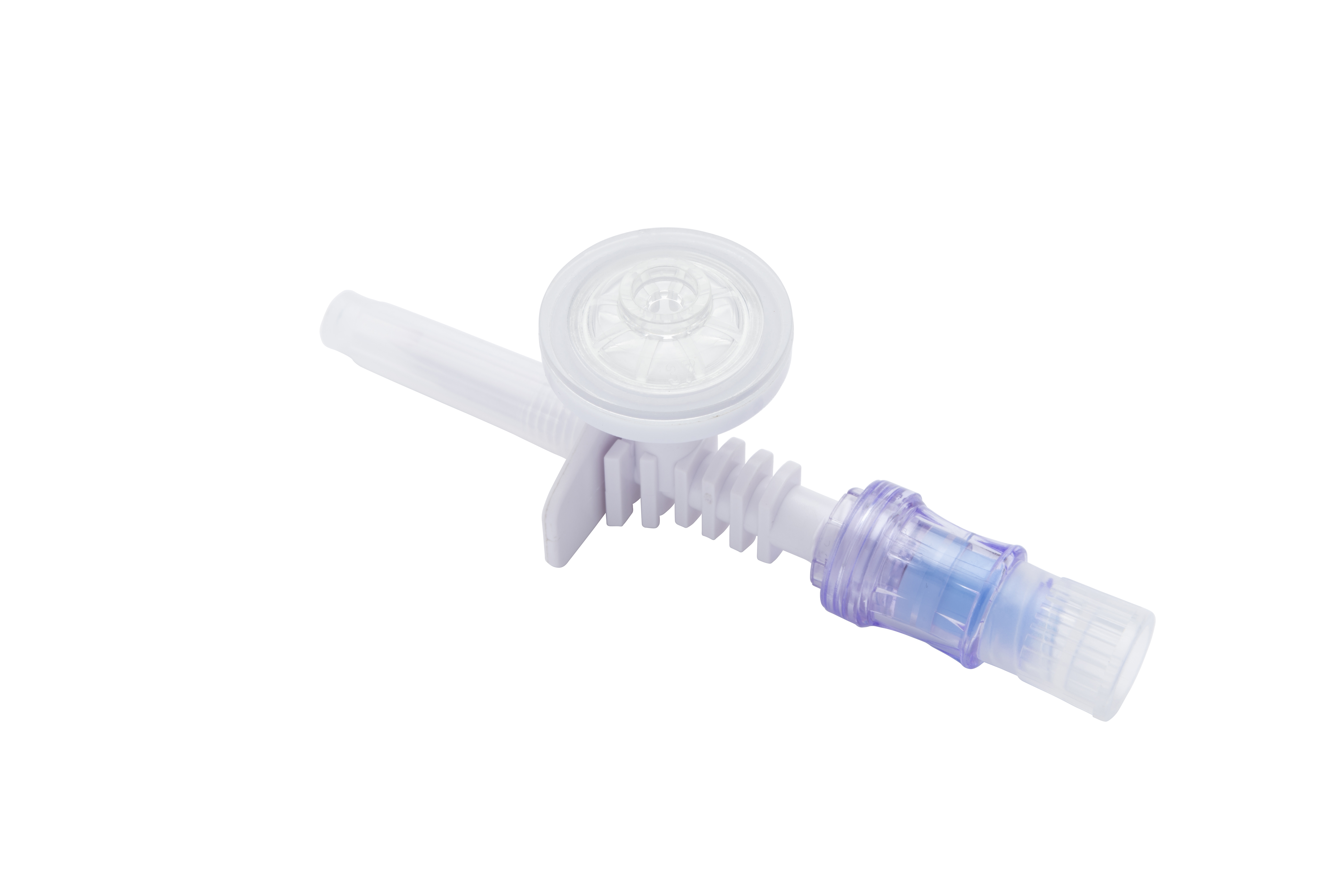
Closed System Vial Adapter Varieties

Light Protected Chemotherapy Drug Administration Sets
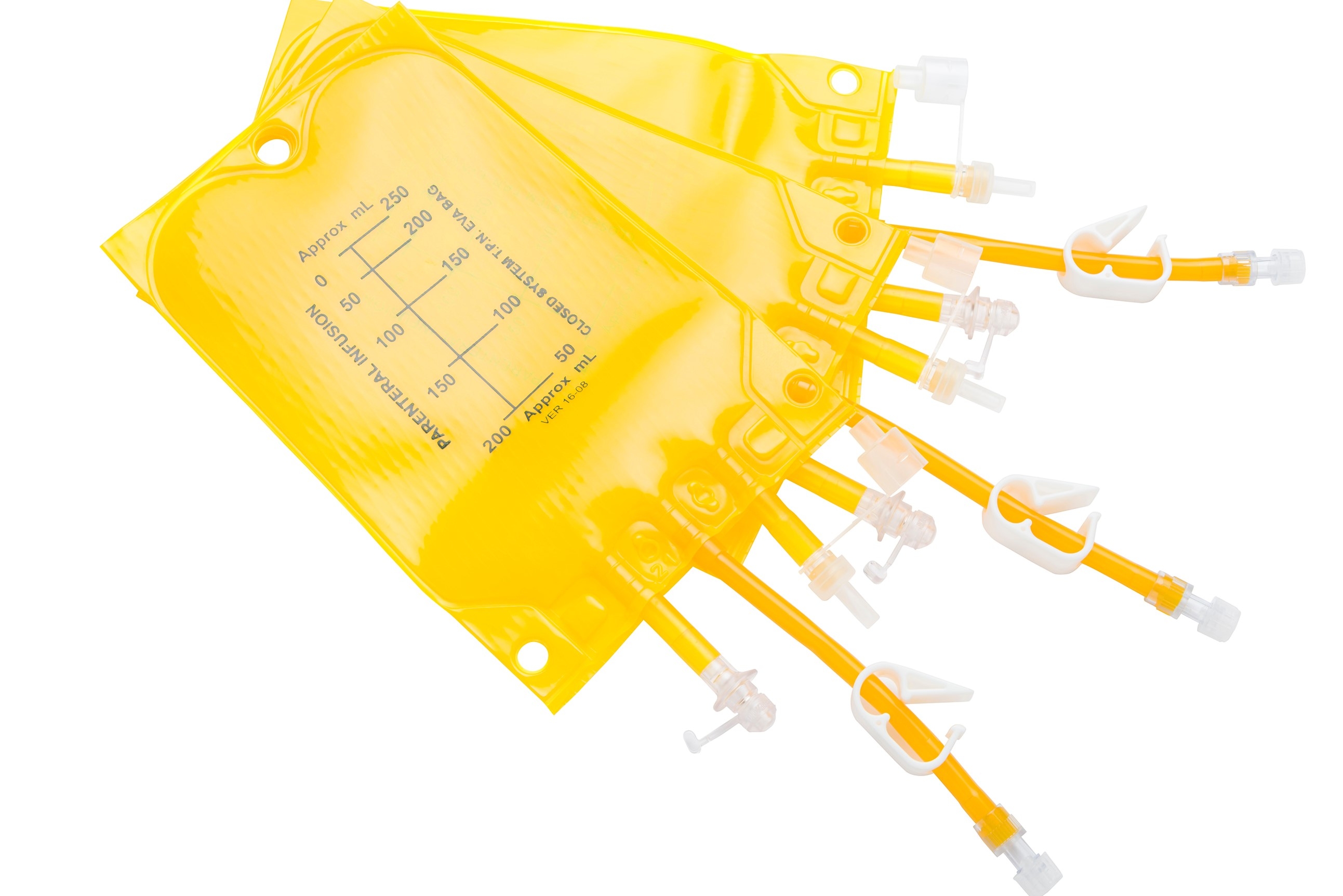
Light Protected Eva Infusion Bags with different port varieties and volumes between 250ml - 5000ml.
No represented companies found.
- ONCOERA

 TR
TR
