| Company Name | Contact Info | Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
LEPZI DIAGNOSTIKA LTD.
Bul. Tsarigradsko Shose 111- B / / Sofia / BULGARIA
|
Contact Info: +359 87 814 1112
|
Hall: 7
Stand: 745E
|
||||||||||||
| Product Groups | ||||||||||||||
|
||||||||||||||
- Company Info
- Products
- Represented Companies
- Company Brands
Lepzi Diagnostika is an innovative company focused on the production of rapid diagnostics tests with Point of Care devices. Leveraging our state-of-the-art immunoassay platform, healthcare professionals can swiftly obtain results in close-to-patient settings. This expedites the decision-making process for patient diagnosis and treatment, ensuring timely and effective medical intervention. Lepzi Diagnostika, a holder of the ISO 13485 certificate, has successfully implemented a Quality Management System (QMS). The company is committed to adhering to all regulations and proudly carries the CE mark, demonstrating its compliance with European standards.

Bladder Tumour Antigen, BTA (also known as Complement Factor H) is a single-use colloidal gold immunoassay intended for qualitative detection of BTA in urine. The test is intended as an aid in the assessment and diagnosis of patients on suspicion of urinary system lesions or bladder cancer in conjunction with cystoscopy and standard hospital procedures. The Lepzi BTA Test is intended for use by individuals trained in point-of-care settings. For professional use only and in patients over/or 18 years of age.

Lepzi PLGF Test is an immunofluorescence in vitro diagnostic test to be used with the Lepzi Reader for the quantitative determination of Placental Growth Factor (PLGF) in EDTA anticoagulated whole blood or plasma specimens. The test is intended for use as an aid in the diagnosis of preterm pre-eclampsia in women presenting with signs and symptoms of pre-eclampsia between 20 and 35 weeks gestation, in conjunction with other diagnostic and clinical information. The Lepzi PLGF Test is intended for use by individuals trained in point-of-care settings and proficient in performing tests using the Lepzi Reader. For professional use only and with patients over 18 years of age.
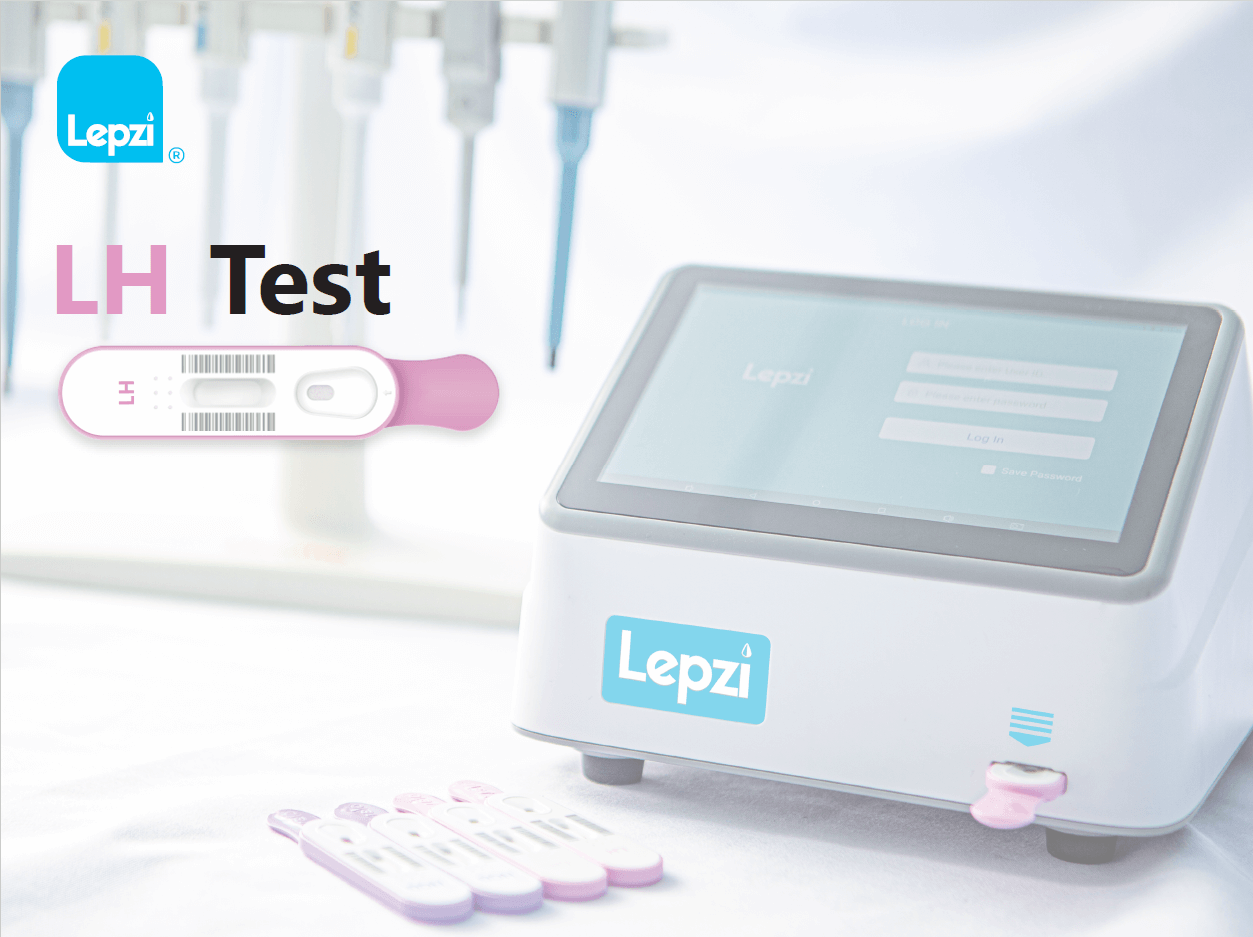
The Lepzi LH test is a colloidal gold In Vitro diagnostic test to be used with the Lepzi Colloidal Gold Analyser for quantitative determination of Luteinising Hormone (LH) in female urine. The test is intended for use to aid in the detection of ovulation. The Lepzi LH Test is intended for use by individuals trained in point-of-care settings and proficient in performing tests using the Lepzi Colloidal Gold Analyser. For professional use only and with patients over 18 years of age.
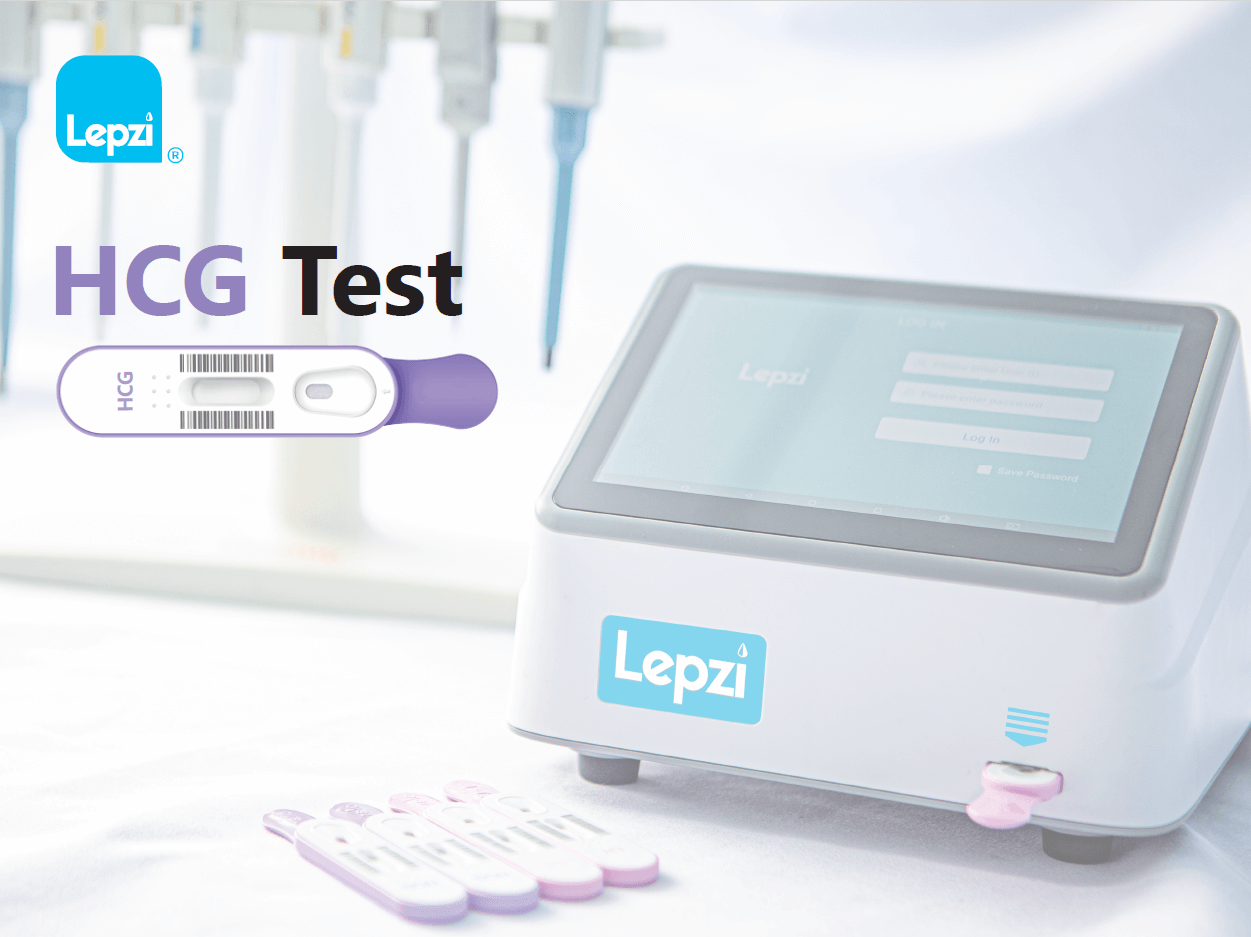
The Lepzi hCG test is an In Vitro diagnostic test to be used with the Lepzi Colloidal Gold Analyser for quantitative determination of human chorionic gonadotrophin (hCG) in female urine. The test is intended for use in pregnancy assessment. The Lepzi hCG Test is intended for use by individuals trained in point-of-care settings and proficient in performing tests using the Lepzi Colloidal Gold Analyser. For professional use only.
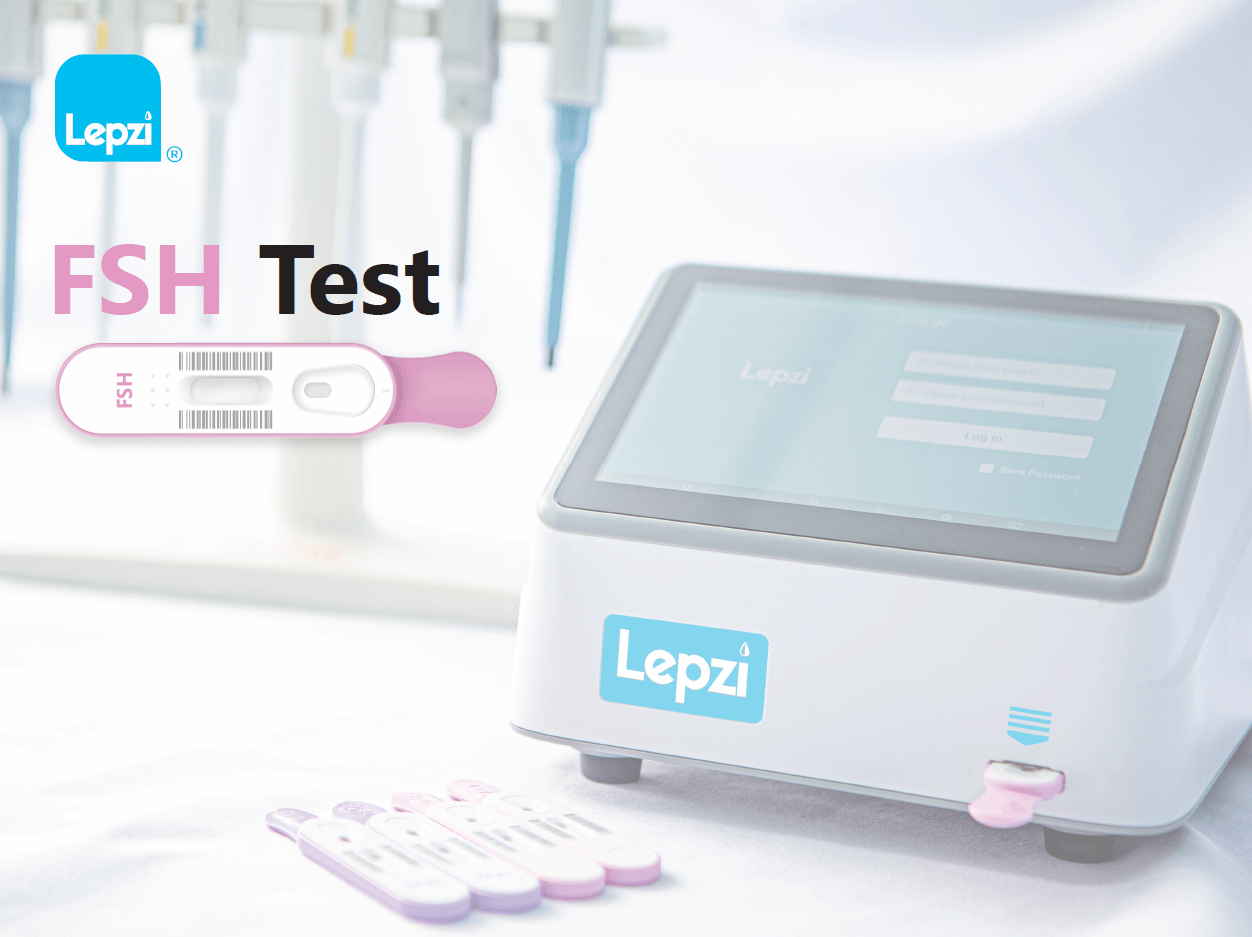
The Lepzi FSH test is a colloidal gold In Vitro diagnostic test to be used with the Lepzi Colloidal Gold Analyser for quantitative determination of Follicle-Stimulating Hormone (FSH) in female urine. The test is intended for use to aid in the diagnosis or evaluation of the female menstrual cycle related to menopause onset and infertility assessment. FSH is a hormone released by the pituitary gland, located on the underside of the brain. In women, FSH helps control the menstrual cycle and the production of eggs by the ovaries. The Lepzi FSH Test is intended for use by individuals trained in point-of-care settings and proficient in performing tests using the Lepzi Colloidal Gold Analyser. For professional use only and with patients over 18 years of age.
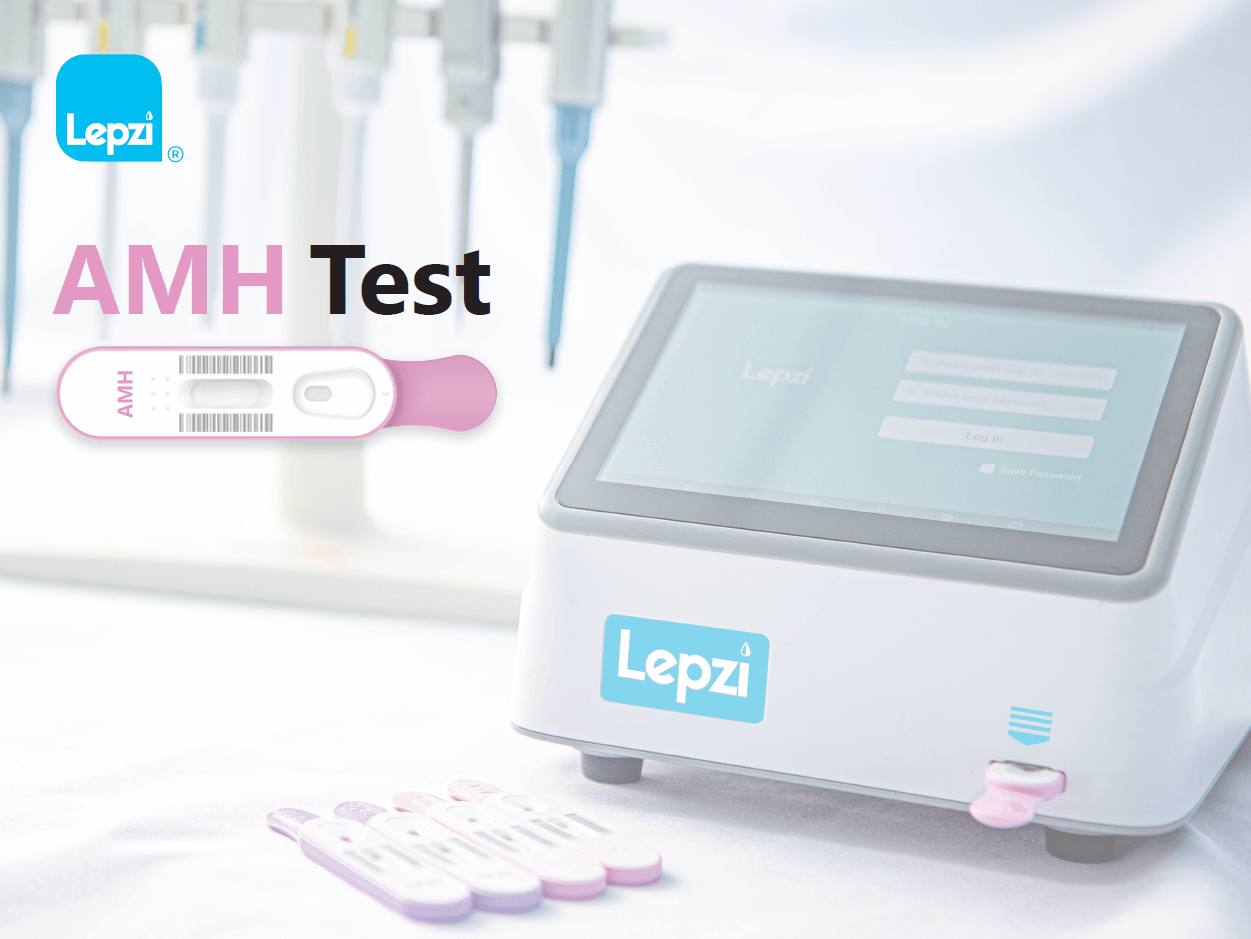
The Lepzi AMH Test is a colloidal gold In Vvitro diagnostic test to be used for the quantitative determination of Anti-Müllerian hormone (AMH) in human serum or plasma. The test is intended for use as an aid in the assessment of ovarian reserve in women attending infertility clinics. The test is intended for use by individuals trained in point-of-care settings and proficient in performing tests using the Lepzi Colloidal Gold Analyser. For professional use only and with patients over 18 years of age.

LEPZI ® Full Portfolio at www.lepzi.com
No represented companies found.
The company has no registered brand information.

 TR
TR
