| Company Name | Contact Info | Location | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABİB FARMA İLAÇ TIBBİ CİH. MED. ORT. GIDA TAR. BİTK. YAĞLAR ÜRT. İNŞ. VE İNŞ. MLZ. SAN. TİC. LTD. ŞTİ.
Sanayi Mahallesi 3327 Sokak Gülpetek Sanayi Sitesi S Ada 3. Blok No: 3 / / ISPARTA / TÜRKİYE
|
Contact Info: +90 533 392 1363
|
Hall: 8
Stand: 825E
|
||||||||||||
| Product Groups | ||||||||||||||
|
||||||||||||||
- Company Info
- Products
- Represented Companies
- Company Brands
Alcasis Genta Bone Cement is a PMMA bone cement available with antibiotics (Gentamicin) in two different viscosities to accommodate surgeons preferences. All our cements meet or exceed the International ISO 5833 Standard for PMMA bone cements. We offer two viscosities for various applications, ensuring a short residence time and efficient application. The packaging is stable and easy to use, with liquid in one ampoule and powder in one Tyvek sachet. Our products are manufactured in accredited facilities, adhering to leading European and international standards. Alcasis Genta Bone Cement Performance Viscosity is a crucial characteristic for surgeons, and we provide both standard and low viscosity bone cements, with or without antibiotics, catering to the diverse needs of surgeons. All our cements conform to the International ISO 5833 PMMA standard, defining physical, mechanical, packaging, and labeling requirements for bone cements. To prevent necrosis of surrounding tissue, International Standard ISO 5833 (2002) sets a curing temperature limit of 90 °C for bone cements. The highest exothermic temperature of Alcasis Genta Cement variants is well below this limit. Our antibiotic bone cements, containing Gentamicin, effectively cover approximately 62% of all gram-negative bacteria. The addition of Gentamicin prevents bacterial adherence to the cement surface and surrounding tissue, reducing the risk of bacterial growth and infection. We limit the antibiotic amount to 2.44% of the total powder volume to ensure effective antibiotics without compromising mechanical strength. Genta Cement offers a high antibiotic release that steadily decreases over time. Burst release initially reduces the risk of resistant bacterial strains, while extended release helps maintain a physiologically relevant local antibiotic concentration for adequate prophylaxis. Each Alcasis Genta Bone Cement package includes a bowl and spatula made from acid-resistant raw materials.
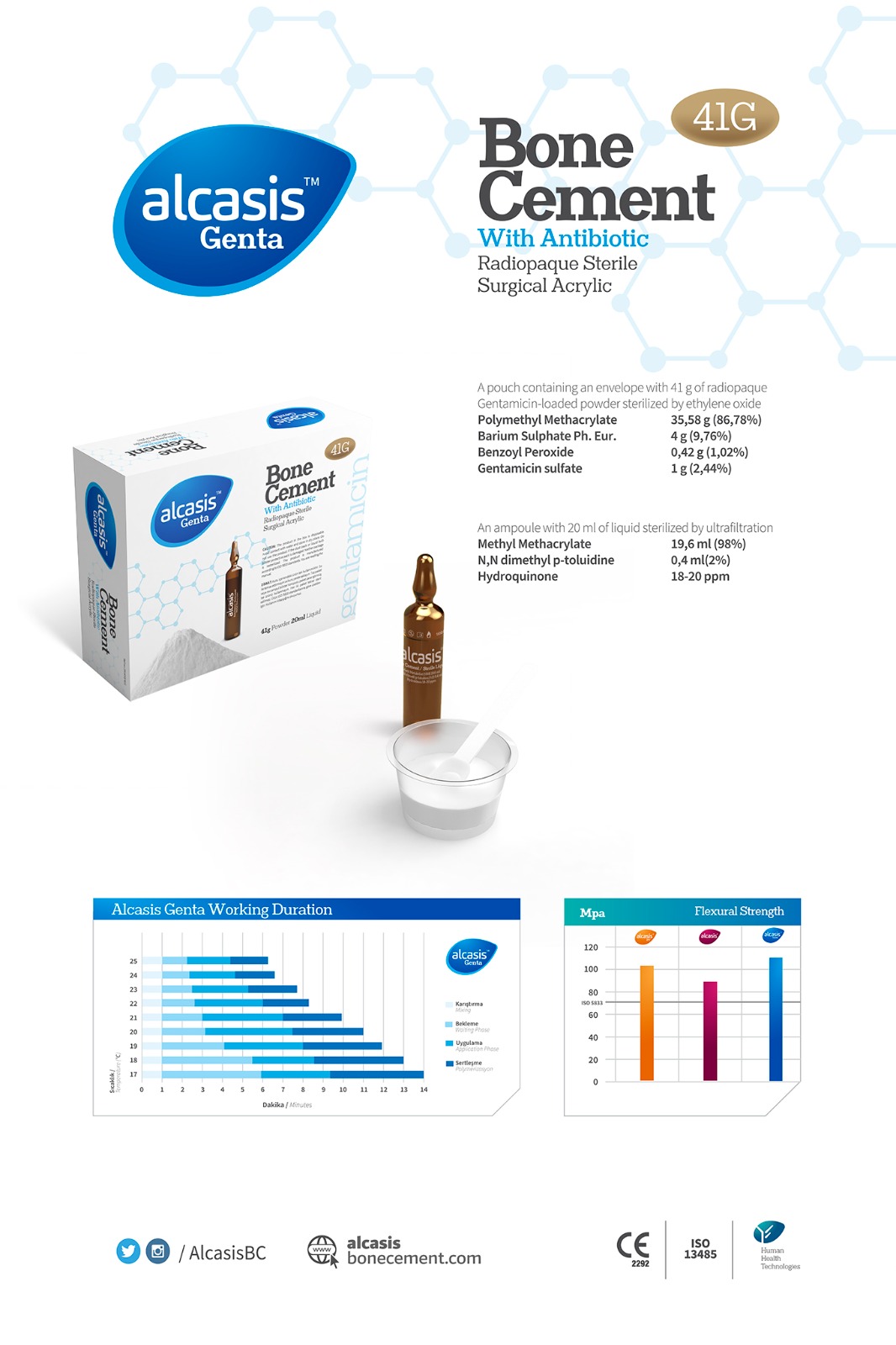
Alcasis Genta Bone Cement is a PMMA bone cement available with antibiotics (Gentamicin) in two different viscosities to accommodate surgeons preferences. All our cements meet or exceed the International ISO 5833 Standard for PMMA bone cements. We offer two viscosities for various applications, ensuring a short residence time and efficient application. The packaging is stable and easy to use, with liquid in one ampoule and powder in one Tyvek sachet. Our products are manufactured in accredited facilities, adhering to leading European and international standards. Alcasis Genta Bone Cement Performance Viscosity is a crucial characteristic for surgeons, and we provide both standard and low viscosity bone cements, with or without antibiotics, catering to the diverse needs of surgeons.
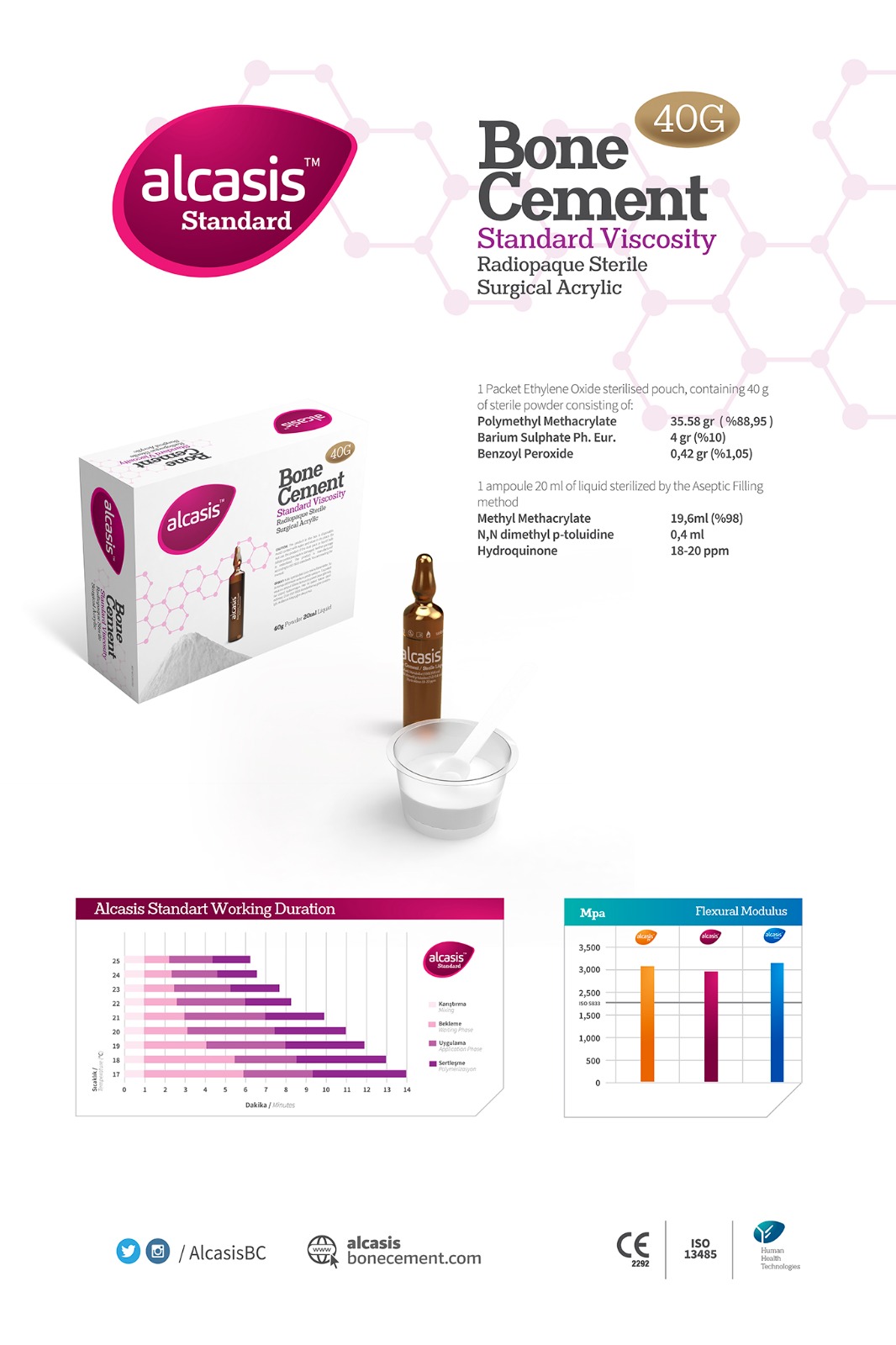
ALCASIS Bone Cement is a medium-viscosity bone cement designed for fixation in hip, knee, and shoulder implants. In orthopedics and various surgical specialties, its application is recommended for securing prosthetic elements using a sterile polymerizable material. Manufactured and tested in accordance with ISO 5833 acrylic resin cement standards, ALCASIS bone cement consistently demonstrates superior quality and mechanical performance across all formulations. ALCASIS bone cement possesses exceptional adhesion and mechanical properties, offering high visibility under X-ray due to the use of Ph Eur BP USP synthetic Barium Sulphate as an opacifier. In terms of application, modern systems are produced for use with syringe/cement gun and manual application. ALCASIS Bone Cement is packaged in a double-layer Tyvek envelope, with the powder portion creating a robust sealing and protective barrier. This design facilitates clean and easy pouring of the cement powder into the preparation container. Additionally, the product is shielded from external factors through blister packaging, ensuring safe delivery to the end-user, the surgical personnel. The formulation provides sufficient working time and appropriate polymerization temperature. All our cements not only meet but also exceed the International ISO 5833 Standard for PMMA bone cements. When utilizing Alcasis bone cement, each product package includes a bowl and spatula made from acid-resistant raw materials, further enhancing the product s practicality and suitability for surgical applications.
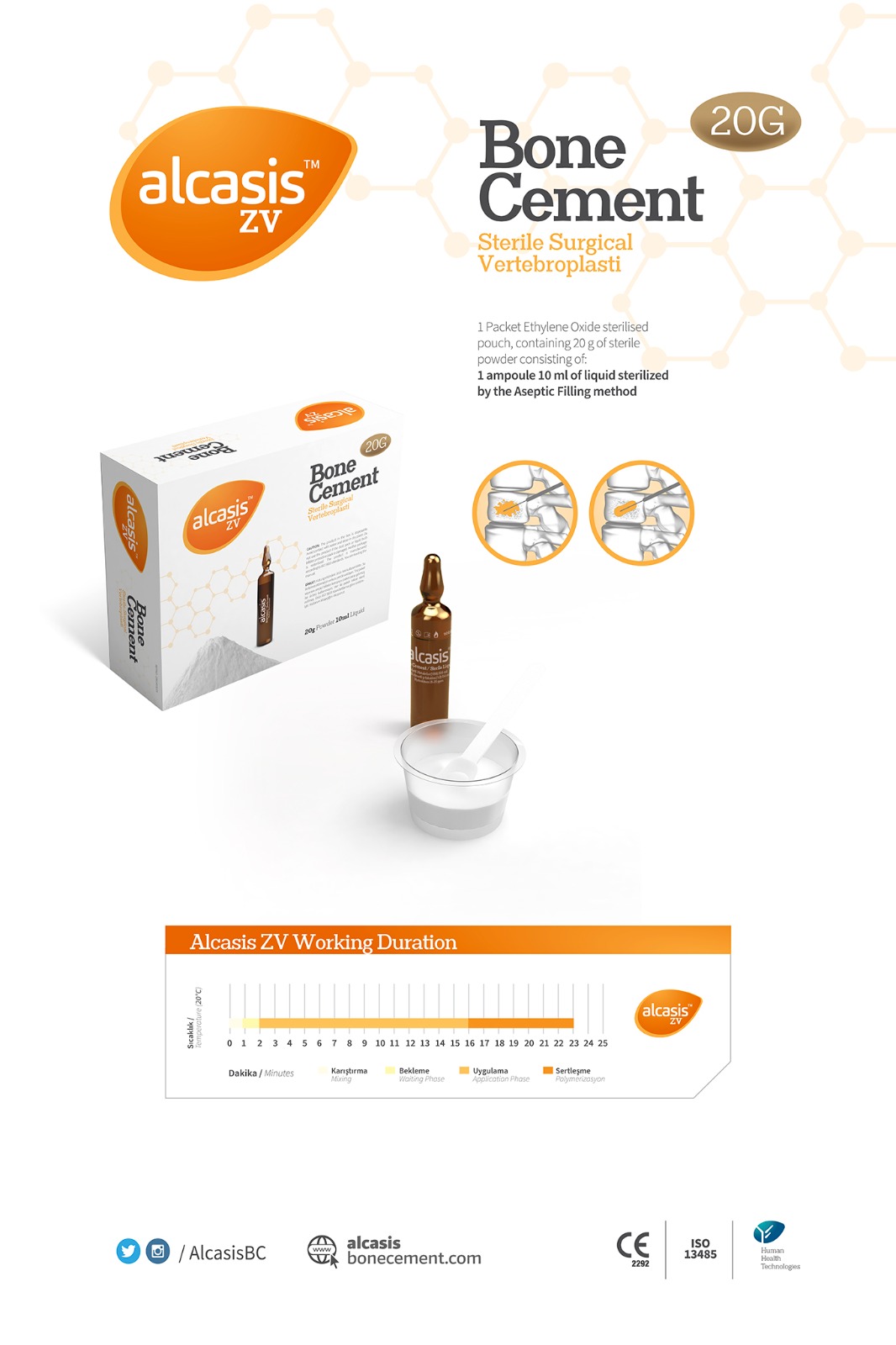
ALCASIS ZV is specifically designed for use in low-viscosity acrylic bone cement, particularly for vertebroplasty and kyphoplasty procedures. It offers sufficient working time and an appropriate polymerization temperature. The inclusion of Ph Eur BP USP synthetic Barium Sulphate as an opacifier ensures high visibility under X-ray. This product is suitable for application in cases of osteoporotic vertebral fractures in patients undergoing spinal angiography. It is indicated for strengthening and stabilizing fractured, collapsed, or compressed spine bodies resulting from pathological conditions such as osteoporosis or tumors. A low reaction rate is a key feature, minimizing exothermic damage to bones or surrounding tissues. The high radiopaque content ensures excellent visibility during procedures. When using Alcasis ZV Vertebroplasty bone cement, each product package includes a bowl and spatula made from acid-resistant raw materials. Clinical performance of Alcasis ZV bone cement is illustrated in it s catalogue.
No represented companies found.
- ALCASİS BONE CEMENT

 TR
TR